Abstract
Mutations in the DNA methyltransferase 3A (DNMT3A) gene are recurrent in de novo acute myeloid leukemia (AML) (20-35%) and are associated with poor prognosis. Although studies demonstrated survival benefit after daunorubicin dose intensification for DNMT3A mutant AML, these patients tend to be older (median age at diagnosis 67 y.o. in DNMT3A-mutated cases compared to 60 y.o. in DNMT3A-WT) and more likely to be unfit for high-dose chemotherapy. In older patients, cytarabine monotherapy, a nucleoside analog chain terminator that stalls DNA replication, may be preferred due to lower toxicity. However, its efficacy in DNMT3A mutant AML has not been evaluated.
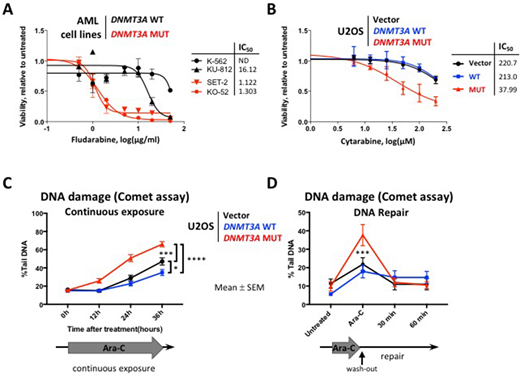
Previous gene expression studies in large AML cohorts and in a mouse model of Dnmt3a-mutant hematopoiesis demonstrated negative enrichment of the cell cycle related signatures, including G2/M checkpoint and E2F targets. Hence, we hypothesized cell cycle-specific drugs cytarabine and fludarabine may be effective in DNMT3A-mutant context. DNMT3A mutant AML cell lines (MUT) SET-2 and KO-52 were more sensitive to cytarabine and fludarabine than DNMT3A wild-type (WT) cells KU-812 and K-562 in vitro (cytarabine IC50 46.1 and 165.8 μM in MUT SET-2 and KO-52 compared to 465.2 μM and not reached in WT KU-812 and K-562, respectively; fludarabine IC50 1.1 and 1.3 μg/ml in MUT vs 16 μg/ml and not reached in WT, Figure 1A). Higher proportion of cells with mutant DNMT3A were undergoing apoptosis 24 hours after cytarabine exposure (26.5±2.3% and 13.1±2.3% Annexin V+ cells in SET-2 and KO-52 vs 6.9±2.6% and 4.6±2.5% in KU-812 and K-562, respectively, p<0.05). Analysis of the DNA damage signaling revealed increased levels of phospho-CHK1, γH2A.X, and cleaved PARP (stalled replication forks, DNA damage, and apoptosis markers).
To investigate the mechanism of differential sensitivity to cytarabine-induced DNA damage, we overexpressed WT or R882 mutant forms of DNMT3A in U2OS cells, a well-established model for DNA damage studies. MUT cells were more sensitive to cytarabine compared to WT (IC50 38 μM vs 213 μM after 48 hours of exposure, Figure 1B), which was accompanied by increased apoptosis (22.3±7.6% in MUT vs 7.2±0.3% in WT Annexin V+ cells, p<0.03). DNMT3A-MUT cells showed persistence of the phospho-CHK1 levels over 12 hours of continuous cytarabine exposure, whereas WT showed resolution of the CHEK1 signaling after initial activation. Consistently, MUT cells accumulated significantly more DNA damage over 36 hours of continuous exposure compared to WT, as evidenced by γH2A.X immunofluorescent staining, and by Comet assay (Figure 1C). Fewer MUT cells were able to complete replication and progress to the G2 phase of the cell cycle (5.3±0.2% in MUT vs 11.2±0.6% in WT, flow-cytometric analysis by DNA content, p<0.01). Notably, both WT and MUT cells were proficient in DNA repair by homologous recombination (HR, tested by RAD51 foci formation visualized by immunofluorescence) and by non-homologous end joining (NHEJ, 53BP1 foci). These data demonstrate that enhanced sensitivity to cytarabine in cells expressing mutant DNMT3A is due to increased susceptibility to DNA damage during replication, and not to defects in DNA repair. Consistently, even though MUT cells showed more DNA damage by Comet assay after 12 hours of cytarabine treatment, it was largely repaired 60 minutes after drug wash-out, similar to WT, Figure 1D). These observations support continuous infusion of cytarabine as a delivery method of choice in the clinic, rather than bolus administration. Finally, bone marrow cells derived from Dnmt3a-mut conditional knock-in mice showed impaired clonogenic survival in MethoCult when exposed to 25 nM cytarabine ex vivo, compared to Dnmt3a-WT (p<0.003). We are currently investigating gene expression, DNA methylation, and chromatin accessibility profiles in WT and MUT cells; and cytarabine efficacy in a pre-clinical mouse model of Dnmt3a(mut):Flt3(ITD):Npm1(c) leukemia.
In conclusion, our studies show cells with DNMT3A mutations may be sensitive to DNA damage induced by clinically relevant nucleoside analogs such as cytarabine. Our data support continuous infusion of cytarabine rather than bolus administration, and establish its mechanistic basis. These results demonstrate, in addition to its role in epigenetic control, DNMT3A contributes to preserving genome integrity during DNA replication.
Licht:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal